Recently, New Gene (Hangzhou) Bioengineering Co., Ltd. (Hereinafter referred to as "NEWGENE")’s novel coronavirus detection products have been registered in British Medicines and Healthcare Products Regulatory Agency (MHRA), and obtained the EU CE certification. NEWGENE is a member on the "Export Allow List" issued by the Chinese Ministry of Commerce for anti-epidemic products, including COVID-19 IgM/IgG Antibody Detection Kit and Novel Coronavirus Spike Glycoprotein Detection Kit.
The principle of antigen detection is to check whether pathogens exist in the human body through stool and sputum samples, while antibody detection focuses on checking if antibodies have been produced against pathogens through blood samples. The two methods are complementary to each other. NEWGENE is one of the few Chinese manufacturers that can export both antibody detection kits and antigen detection kits, providing customized and professional solutions for various needs.
NEWGENE is located in Hangzhou Tianhe High-tech Industrial Park, a high-tech company engaged in the research, development, manufacture and distribution of biological products. It is committed to creating biological materials such as antigens and antibodies, in vitro diagnostic reagents and related devices, and also the entire industrial chain layout of artificial intelligence assisted diagnosis system. The company's product line covers a full range of in vitro diagnostic products such as immune diagnosis, molecular diagnosis, and microbiological testing. It has profound technical accumulation and unique technological advantages in the areas of early cancer screening, rapid detection of infectious diseases, and rapid screening of geriatric diseases.

MHRA Registration Letter

CE Declaration of Conformity
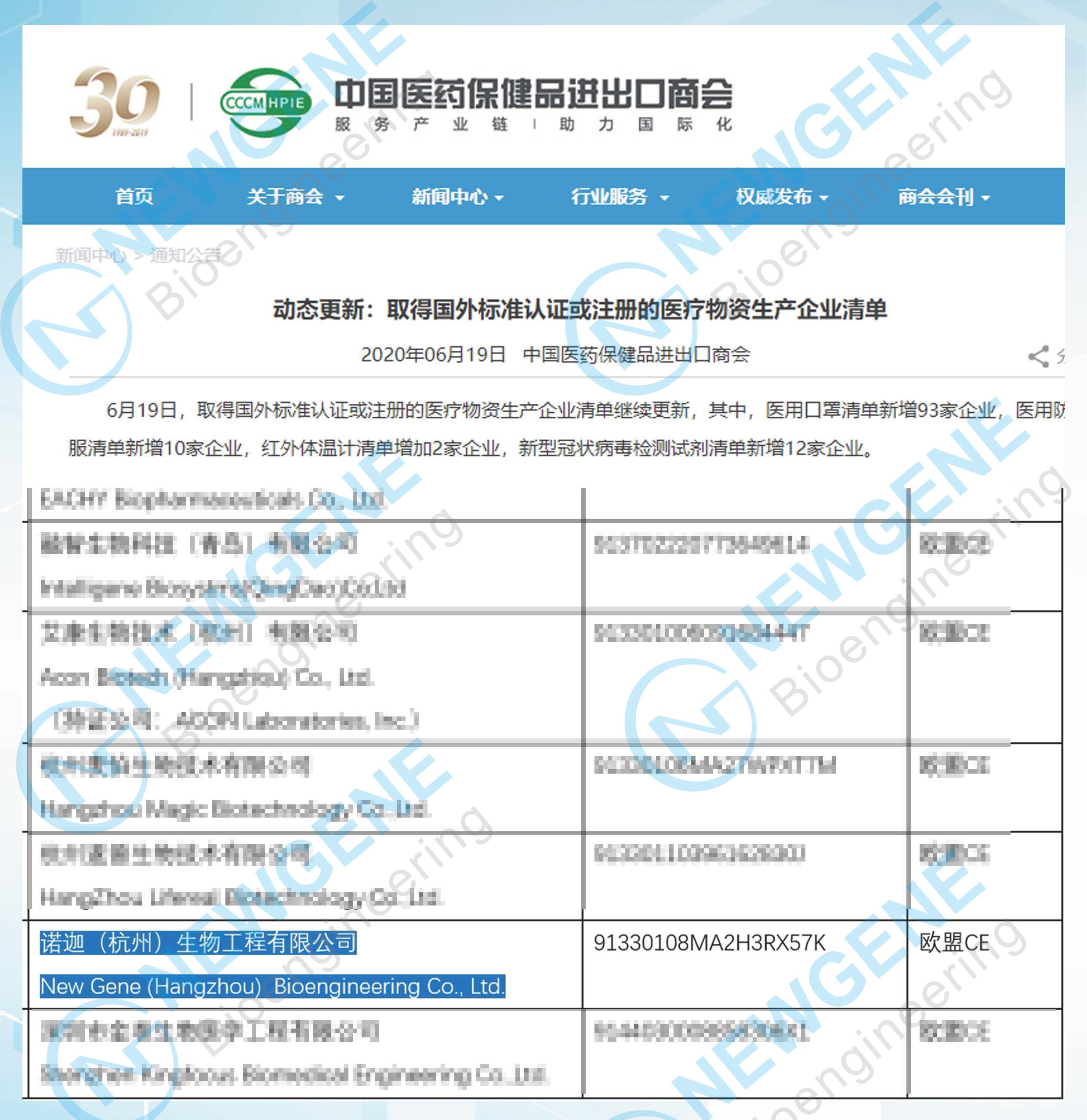
Screenshot of the "Export Allow List" issued by the Chinese Ministry of Commerce for anti-epidemic products

GMP Manufacture Site
 中文
中文 English
English

 WeChat official account
WeChat official account